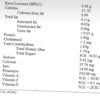
Iron content of Kava.
Today’s fact of the day will be dealing with the content of kava not necessarily related to kavalactones. I’ve seen a few posts recently regarding the iron content of kavas, and this could possibly shed some light on the situation. Kava, as a natural product, contains an array of minerals and other compounds we see in plant material. Dried kava was found to have 43% starch, 20% fiber, 12% water, 3.2% sugars, 3.6% proteins, 3.2% minerals with between 3% and 20% kavalactones by weight [1].
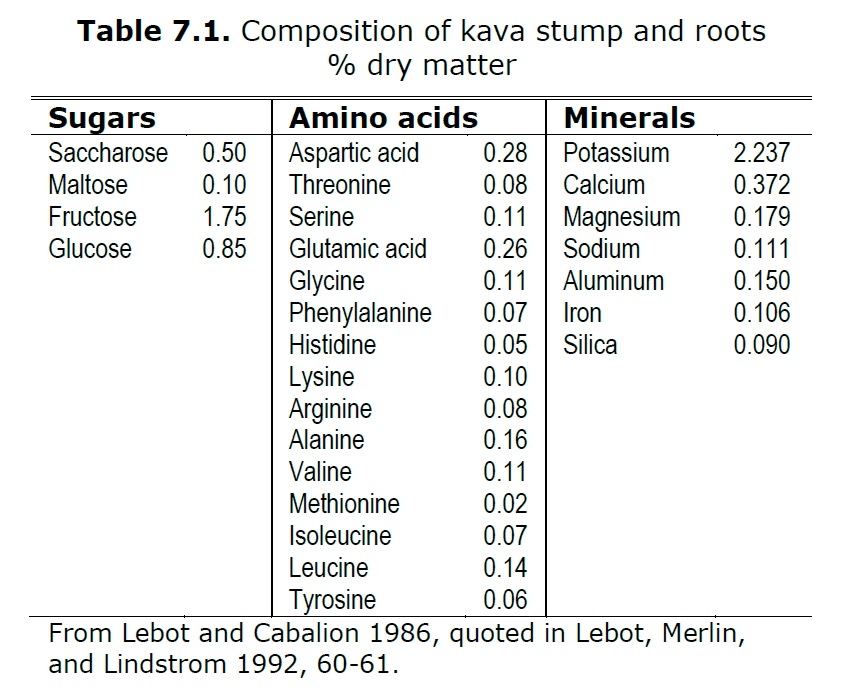 In figure 7.1 above you can see the breakdown of the constituents of kava. I would like to focus our attention on the minerals column, and specifically iron [1]. Iron is an extremely important mineral in the life cycle of plants. Plants utilize iron in the creation of chlorophyll and is essential for the maintenance of chloroplast structure and function [2]. Given iron’s functional role in plant life, it would make sense that we also see it in our prepared kavas. Several kava drinkers have brought up issues of finding what looks like iron filings in their kavas after preparation and settling. The jury is still out on it’s source, however I propose here that one possible source is the kava itself. Going back to the table above we see that the iron content in kava was found to be .106%. This comes out to somewhere around
In figure 7.1 above you can see the breakdown of the constituents of kava. I would like to focus our attention on the minerals column, and specifically iron [1]. Iron is an extremely important mineral in the life cycle of plants. Plants utilize iron in the creation of chlorophyll and is essential for the maintenance of chloroplast structure and function [2]. Given iron’s functional role in plant life, it would make sense that we also see it in our prepared kavas. Several kava drinkers have brought up issues of finding what looks like iron filings in their kavas after preparation and settling. The jury is still out on it’s source, however I propose here that one possible source is the kava itself. Going back to the table above we see that the iron content in kava was found to be .106%. This comes out to somewhere around 10.6mg 106mg per 100g of kava (thank you, Shulgin46 from reddit for the correction). If we look through research about iron fortification in common food items, we find that we can see an elemental iron amount as high as 14mg per 100g of standard breakfast cereals available on the US market [3]. An experiment was formulated to visually identify the iron in these cereals by simply crushing the flakes, spreading them over a surface, and running a magnet under it to see what is collected [4]. It was found that elemental metallic iron would be pulled out by the magnet to an extent that it was identifiable by vision alone. It’s important to understand that metallic iron, while it seems like something you wouldn’t want to eat, is actually around 13% bioavailable for absorption into the body [3], and used quite commonly to fortify foods. Another source could be due to the hammer action of the hammer mills when grinding the kava, however if this amount of iron were to be in every batch of kava from the mill, they would be replacing the hammers after every batch because they would become ineffective quickly if they were shedding that much mass in iron. Blades are normally changed once per year. It looks, based on research, that the wear of hardened steel blades wouldn't necessarily even account for that much iron in kava [5].
In summary, while we can be confident that the black sediment at the bottom of the kava bowl is likely iron, the jury is still out on it’s source. The amount of iron present is not harmful, and seems to roughly bethe amount found in fortified cereals about 10 times what is found breakfast cereals. I propose here that kava itself is the source, however more testing and observation is necessary to elucidate the confirmed source of this metallic iron.
[1] Klaus Dragull Jim Henderson Mel C. Jackson Ed Johnston Jerry Konanui G. David Lin Kepā Maly Scot Nelson Jeri Ooka Tom Osborn Helen Rogers Chung-Shih Tang. 2006. Hawaiian ‘Awa Views of an Ethnobotanical Treasure. Edited by Ed Johnston and Helen Rogers. Association for Hawaiian ‘Awa.
https://gourmethawaiiankava.com/hawaiian-awa-views-of-an-ethnobotanical-treasure
[2] Rout, Gyana R., and Sunita Sahoo. 2015. “ROLE OF IRON IN PLANT GROWTH AND METABOLISM.” Reviews in Agricultural Science 3: 1–24.
https://doi.org/10.7831/ras.3.1.
[3] Lermyte, Frederik, Wen-Ying Zhang, Jake Brooks, Steven Huband, Joanna F. Collingwood, Martin R. Lees, Margaret P. Rayman, and Peter J. Sadler. 2020. “Metallic Iron in Cornflakes.” Food & Function 11 (4): 2938–42.
https://doi.org/10.1039/c9fo02370d.
[4] Maynard, J., & Jacobsen, E. K. (2004). Iron in Breakfast Cereal. Demonstrations for National Chemistry Week 2004. Journal of Chemical Education, 81(11), 1544. doi:10.1021/ed081p1544
https://sci-hub.st/10.1021/ed081p1544
[5] Roy, Sougata, Kyungjun Lee, Jeffrey A. Lacey, Vicki S. Thompson, James R. Keiser, and Jun Qu. 2020. “Material Characterization-Based Wear Mechanism Investigation for Biomass Hammer Mills.” ACS Sustainable Chemistry & Engineering 8 (9): 3541–46.
https://doi.org/10.1021/acssuschemeng.9b06450.
Today’s fact of the day will be dealing with the content of kava not necessarily related to kavalactones. I’ve seen a few posts recently regarding the iron content of kavas, and this could possibly shed some light on the situation. Kava, as a natural product, contains an array of minerals and other compounds we see in plant material. Dried kava was found to have 43% starch, 20% fiber, 12% water, 3.2% sugars, 3.6% proteins, 3.2% minerals with between 3% and 20% kavalactones by weight [1].
In summary, while we can be confident that the black sediment at the bottom of the kava bowl is likely iron, the jury is still out on it’s source. The amount of iron present is not harmful, and seems to roughly be
[1] Klaus Dragull Jim Henderson Mel C. Jackson Ed Johnston Jerry Konanui G. David Lin Kepā Maly Scot Nelson Jeri Ooka Tom Osborn Helen Rogers Chung-Shih Tang. 2006. Hawaiian ‘Awa Views of an Ethnobotanical Treasure. Edited by Ed Johnston and Helen Rogers. Association for Hawaiian ‘Awa.
https://gourmethawaiiankava.com/hawaiian-awa-views-of-an-ethnobotanical-treasure
[2] Rout, Gyana R., and Sunita Sahoo. 2015. “ROLE OF IRON IN PLANT GROWTH AND METABOLISM.” Reviews in Agricultural Science 3: 1–24.
https://doi.org/10.7831/ras.3.1.
[3] Lermyte, Frederik, Wen-Ying Zhang, Jake Brooks, Steven Huband, Joanna F. Collingwood, Martin R. Lees, Margaret P. Rayman, and Peter J. Sadler. 2020. “Metallic Iron in Cornflakes.” Food & Function 11 (4): 2938–42.
https://doi.org/10.1039/c9fo02370d.
[4] Maynard, J., & Jacobsen, E. K. (2004). Iron in Breakfast Cereal. Demonstrations for National Chemistry Week 2004. Journal of Chemical Education, 81(11), 1544. doi:10.1021/ed081p1544
https://sci-hub.st/10.1021/ed081p1544
[5] Roy, Sougata, Kyungjun Lee, Jeffrey A. Lacey, Vicki S. Thompson, James R. Keiser, and Jun Qu. 2020. “Material Characterization-Based Wear Mechanism Investigation for Biomass Hammer Mills.” ACS Sustainable Chemistry & Engineering 8 (9): 3541–46.
https://doi.org/10.1021/acssuschemeng.9b06450.
Last edited: