What is HPLC?
HPLC is short for “High-performance liquid chromatography”. This is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. HPLC is used in manufacturing, research, and medical fields [1]. In regards to kava, HPLC is used to separate and quantify the different kavalactones in a sample [2].
How does HPLC work?
In HPLC a pump forces a solvent mixed with the testing sample through a metal cylinder that is packed full of adsorbent material called a “column”. Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid, or dissolved state to a surface [3]. At the outgoing end of the metal tube or “column” there will be placed a detector which computes sample quantities and specific molecules [4]. This process is highly automated and very sensitive.
Retention Time.
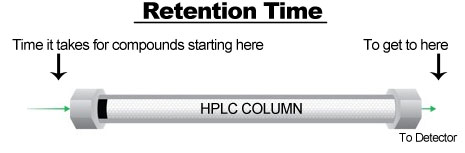
Retention time is a measure of time that it takes for the sample and solvent to enter from one side of the column to reach the detector at the opposite end of the column. Each compound will have differing retention times based on its composition [5].
Kava Chemotype.
Chemotype is responsible for the quality of kava’s physiological effect. When we refer to chemotypes we express them in 6 digit strings. For example the variety “Kelai” from Vanuatu is seen as having a 423156 chemotype. Each one of the digits corresponds to a different kavalactone in a descending ratio. What this means is that numbers towards the front of the 6 digit string will be higher in quantity than those at the end. A note to keep in mind is that this only represents their relative proportion to each other, and the numbers can vary from each other by only fractions of a percent [6].
How the different kavalactones got their numbers.

It’s been said that these numbers were given arbitrarily by researchers, however this is certainly not the case. Each kavalactone was assigned it’s number based on the increasing time it took for it to exit the column in an HPLC system (Retention Time). It sounds complicated, but it is quite simple. Each kavalactone took a different amount of time to travel the length of the column and that time is how each kavalactone got their number [7]. Desmethoxyyangonin was the quickest kavalactone to exit the column, hence the number 1, where Methysticin took the longest and was assigned the number 6.
[1] High-performance liquid chromatography. (2021). In Wikipedia.
https://en.wikipedia.org/w/index.php?title=High-performance_liquid_chromatography
[2] Lasme, Privat, Fabrice Davrieux, Didier Montet, and Vincent Lebot. 2008. “Quantification of Kavalactones and Determination of Kava (Piper Methysticum) Chemotypes Using near-Infrared Reflectance Spectroscopy for Quality Control in Vanuatu.” Journal of Agricultural and Food Chemistry 56 (13): 4976–81.
https://doi.org/10.1021/jf800439g.
[3] Adsorption. (2021). In Wikipedia.
https://en.wikipedia.org/w/index.php?title=Adsorption
[4] Waters corporation: The science of what’s possible. (n.d.). Retrieved June 10, 2021, from
https://www.waters.com/waters/en_US/How-Does-High-Performance-Liquid-Chromatography-Work
[5] Chromatography Today. (2014, August 1). Understanding the Difference Between Retention Time and Relative Retention Time. Chromatography Today.
https://www.chromatographytoday.com...ention-time-and-relative-retention-time/31166
[6] Lebot, V., T. K. T. Do, and L. Legendre. 2014. “Detection of Flavokavins (A, B, C) in Cultivars of Kava (Piper Methysticum) Using High Performance Thin Layer Chromatography (HPTLC).” Food Chemistry 151 (May): 554–60.
https://doi.org/10.1016/j.foodchem.2013.11.120
[7] Lebot, V., and J. Lèvesque. 1989. “THE ORIGIN AND DISTRIBUTION OF KAVA (PIPER METHYSTICUM FORST. F., PIPERACEAE): A PHYTOCHEMICAL APPROACH.” Allertonia 5 (2): 223–81.
http://www.jstor.org/stable/23187398
HPLC is short for “High-performance liquid chromatography”. This is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. HPLC is used in manufacturing, research, and medical fields [1]. In regards to kava, HPLC is used to separate and quantify the different kavalactones in a sample [2].
How does HPLC work?
In HPLC a pump forces a solvent mixed with the testing sample through a metal cylinder that is packed full of adsorbent material called a “column”. Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid, or dissolved state to a surface [3]. At the outgoing end of the metal tube or “column” there will be placed a detector which computes sample quantities and specific molecules [4]. This process is highly automated and very sensitive.
Retention Time.
Retention time is a measure of time that it takes for the sample and solvent to enter from one side of the column to reach the detector at the opposite end of the column. Each compound will have differing retention times based on its composition [5].
Kava Chemotype.
Chemotype is responsible for the quality of kava’s physiological effect. When we refer to chemotypes we express them in 6 digit strings. For example the variety “Kelai” from Vanuatu is seen as having a 423156 chemotype. Each one of the digits corresponds to a different kavalactone in a descending ratio. What this means is that numbers towards the front of the 6 digit string will be higher in quantity than those at the end. A note to keep in mind is that this only represents their relative proportion to each other, and the numbers can vary from each other by only fractions of a percent [6].
How the different kavalactones got their numbers.
It’s been said that these numbers were given arbitrarily by researchers, however this is certainly not the case. Each kavalactone was assigned it’s number based on the increasing time it took for it to exit the column in an HPLC system (Retention Time). It sounds complicated, but it is quite simple. Each kavalactone took a different amount of time to travel the length of the column and that time is how each kavalactone got their number [7]. Desmethoxyyangonin was the quickest kavalactone to exit the column, hence the number 1, where Methysticin took the longest and was assigned the number 6.
[1] High-performance liquid chromatography. (2021). In Wikipedia.
https://en.wikipedia.org/w/index.php?title=High-performance_liquid_chromatography
[2] Lasme, Privat, Fabrice Davrieux, Didier Montet, and Vincent Lebot. 2008. “Quantification of Kavalactones and Determination of Kava (Piper Methysticum) Chemotypes Using near-Infrared Reflectance Spectroscopy for Quality Control in Vanuatu.” Journal of Agricultural and Food Chemistry 56 (13): 4976–81.
https://doi.org/10.1021/jf800439g.
[3] Adsorption. (2021). In Wikipedia.
https://en.wikipedia.org/w/index.php?title=Adsorption
[4] Waters corporation: The science of what’s possible. (n.d.). Retrieved June 10, 2021, from
https://www.waters.com/waters/en_US/How-Does-High-Performance-Liquid-Chromatography-Work
[5] Chromatography Today. (2014, August 1). Understanding the Difference Between Retention Time and Relative Retention Time. Chromatography Today.
https://www.chromatographytoday.com...ention-time-and-relative-retention-time/31166
[6] Lebot, V., T. K. T. Do, and L. Legendre. 2014. “Detection of Flavokavins (A, B, C) in Cultivars of Kava (Piper Methysticum) Using High Performance Thin Layer Chromatography (HPTLC).” Food Chemistry 151 (May): 554–60.
https://doi.org/10.1016/j.foodchem.2013.11.120
[7] Lebot, V., and J. Lèvesque. 1989. “THE ORIGIN AND DISTRIBUTION OF KAVA (PIPER METHYSTICUM FORST. F., PIPERACEAE): A PHYTOCHEMICAL APPROACH.” Allertonia 5 (2): 223–81.
http://www.jstor.org/stable/23187398
