- maybe a little off topic but have you considered using a VPN? I think all who value liberty should use a VPN to hide their internet activity from snooping authorities as well as allowing access to the whole web without annoying filters.I'm staying in one of my hometown college dorm rooms (because there are no month to month rentals around here) while I await my start date for the federal job i applied for after finishing my degree, and using their internet, kavaforums.com is blocked because it is a drug of abuse according to the message on my screen. The mindset of many people is still stuck way in the past and/or they believe any propaganda they are spoon fed by our so called anti biased main media.
Do you want kava to stay legal? DO SOMETHING!
- Thread starter ApéroNoble
- Start date
Chronophan
Kava Curious
What is happening now to our freedoms would be unthinkable when our country was less prosperous , but some people do not do well without adversity, and seek out demons where there are none. I grew up years ago in the American upper Midwest where the summers were stifling and the winters, deadly. In that world where nature was ready and willing to kill you, people were friendlier, and seemed more accepting of each other's interests. Kids could adventure off by themselves because their parents were at work struggling to make a living. Now, with everything much "safer", and everyone having more free time on their hands, people seem more interested in getting into each other people's business. Society we can slow this vigilantly "there aught to be a law!" mentality long enough to just get used to prosperity, we might actually be able to just enjoy our world without having to make everything anyone else does illeagal.
That's already the case, it's a FDA requirement, although not all vendors comply.I didn't read the whole thread so sorry if someone already post this...
Maybe if the vendors put some nutritional informations on their stickers it will go in the right direction.
Nutritional information is not required if sales do not exceed 100,000 units and the number of employees is less than 100.That's already the case, it's a FDA requirement, although not all vendors comply.
Kalm with Kava
Kava Vendor
I would definitely consult with the FDA to get clarification. As I understand the exemption may not apply to imported products which need to follow cGMP (which is waaaaay more intensive than just the nutritional info) and includes labeling.
https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm238182.htm
Yes. The DS CGMP rule applies to you if you manufacture, package, label, or hold a dietary supplement imported or offered for import in any State or Territory of the United States, the District of Columbia, or the Commonwealth of Puerto Rico.
(21 CFR 111.1(a)(2))
Sent from my VS995 using Tapatalk
In case anyone finds this interestingI would definitely consult with the FDA to get clarification.
...
Sent from my VS995 using Tapatalk
 here's a screenshot of the nutrient labeling exemption application page. At the bottom, there's another statement that if the total number of units is less than 10,000 and the business employs less than 10 people, an exemption application is not even necessary...for food sales.
here's a screenshot of the nutrient labeling exemption application page. At the bottom, there's another statement that if the total number of units is less than 10,000 and the business employs less than 10 people, an exemption application is not even necessary...for food sales.Since the Dietary Supplement Health and Education Act (DSHEA, 1994) defined dietary supplements as a category of food, I am pretty sure small kava businesses that act as distributors or packers (but not importers) are qualified to apply for this exemption. To be 100% sure, I emailed the FDA's small business unit. I'll report back when I find out more.
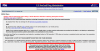
The FDA confirmed that kava vendors can apply for nutritional labeling exemptions as long as they are just distributing and not importing. Also, very small operations are not required to provide nutritional labeling.

Feel Good, Drink Fresh.
www.fijifreshkava.com

Feel Good, Drink Fresh.
www.fijifreshkava.com
I assure you, we find these sorts of things very interesting. Thank you for the follow up. This actually clears up a good bit.In case anyone finds this interestinghere's a screenshot of the nutrient labeling exemption application page. At the bottom, there's another statement that if the total number of units is less than 10,000 and the business employs less than 10 people, an exemption application is not even necessary...for food sales.
Since the Dietary Supplement Health and Education Act (DSHEA, 1994) defined dietary supplements as a category of food, I am pretty sure small kava businesses that act as distributors or packers (but not importers) are qualified to apply for this exemption. To be 100% sure, I emailed the FDA's small business unit. I'll report back when I find out more.
View attachment 9525
Right, no arguments there. I was just talking about the nutrition labeling.@FijiFreshKava this may be true regarding just the labelling requirement, but I think @Kalmwithkava was talking about the requirement to operate in a cGMP facility.
