Today’s fact of the day will highlight an interesting disparity between how different regulatory bodies interpreted data related to the liver injury reports from the early 2000s. BfArM in Germany is the “Federal Institute for Drugs and Medical Devices”. This is the regulatory agency responsible for the kava ban which swept the world as other countries looked to Germany for direction. We’re going to take a look at one paper which focuses on four of these instances and how different each regulatory body saw the data as it was presented.
We’ll be addressing the following four specific instances;
(CD = Coadministered Drugs)
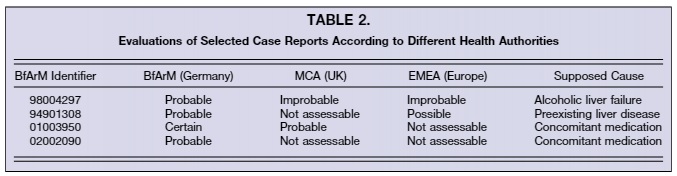
These cases were seen rather differently by each agency [2]. Table 2 shows the differing conclusions between countries and their scientific regulatory bodies. Where BfArM found probable and certain links to kava, other agencies found little to no evidence of kava being the key factor in these injury reports. This is only a small snapshot of the indiscrepancies in interpretation, and highlights the non-agreement between scientific bodies in regards to harm caused by kava. This brings even more questionability in the decision to ban kava products in Germany, which continues to harm the South Pacific to this day.
[1] Teschke, Rolf. 2010. “Kava Hepatotoxicity--a Clinical Review.” Annals of Hepatology 9 (3): 251–65. https://doi.org/10.1016/S1665-2681(19)31634-5.
[2] Gruenwald, Joerg, and Juergen Skrabal. 2003. “Kava Ban Highly Questionable: A Brief Summary of the Main Scientific Findings Presented in the ‘in Depth Investigation on EU Member States Market Restrictions on Kava Products.’” Seminars in Integrative Medicine 1 (4): 199–210. https://doi.org/10.1016/j.sigm.2004.01.001.
We’ll be addressing the following four specific instances;
(CD = Coadministered Drugs)
- Case # 98004297
- Ethanolic kava extract (120 mg/d, 10 m) for anxiety and restlessness. Prolonged kava treatment. Hydrochlorothiazide and Crataegus extract as CD. No ultrasound results. No exclusion of hepatitis A and C, CMV, EBV, HSV, and VZV, but LKM positive. Recurrent ALT increase despite kava cessation, suggesting kava independent cause. No further ALT follow up due to early death. Chronic pancreatitis at autopsy. Lethal outcome. Causality excluded for kava and excluded for CD. Diagnosis: LKM positive AIH and pancreatitis [1].
- Case # 94901308
- Acetonic kava extract (210 mg/d, 1.5 m) for unknown indication. Daily kava overdose. Furosemide, Atenolol, and Terfenadine as CD. Known Terfenadine overdose with up to 300 mg daily (allowed maximal 120 mg). ALT 950 U/L, AST 1045 U/L, and ALP 315 U/L. ALT normalisation not documented after 2 months. Kava unrelated cause due to recurrent increase of ALT. Complete exclusion diagnostic work-up, but HSV-IgM positive. Cessation of initial steroid treatment at time when HSV hepatitis was diagnosed. Favourable outcome. Causality excluded for kava and excluded for CD. Diagnosis: HSV-hepatitis [1].
- Case # 01003950
- No data available. Undeclared indication. Unassessable case. Unclear case, not suitable as index case for a possible subsequent re-administration (see case 10, identical patient). ALT, AST, and ALP not recorded. Favourable outcome. Causality excluded for kava and not assessable for CD. Diagnosis: Questionable liver disease of unknown etiology [1].
- Case # 02002090
- Ethanolic kava extract (50 mg/d, 0.25 m) for stress. Sulfasalazine, Diclofenac, Progesterone, Omeprazole, Butylscopolaminium-bromide as CD. ALT 572 U/L, AST 220 U/L, and ALP 163 U/L. ALT course poorly documented. Exclusion of hepatitis A, B, C, CMV, and EBV mentioned but specific data not documented AMA, SLA/LP, and LKM with negative results, but ANA not reported. Known Bechterew’s disease and adipositas as co-morbidity. Favourable outcome. Causality possible for kava and unlikely for CD. Diagnosis: Liver disease of unknown etiology, possibly related to kava [1].
These cases were seen rather differently by each agency [2]. Table 2 shows the differing conclusions between countries and their scientific regulatory bodies. Where BfArM found probable and certain links to kava, other agencies found little to no evidence of kava being the key factor in these injury reports. This is only a small snapshot of the indiscrepancies in interpretation, and highlights the non-agreement between scientific bodies in regards to harm caused by kava. This brings even more questionability in the decision to ban kava products in Germany, which continues to harm the South Pacific to this day.
[1] Teschke, Rolf. 2010. “Kava Hepatotoxicity--a Clinical Review.” Annals of Hepatology 9 (3): 251–65. https://doi.org/10.1016/S1665-2681(19)31634-5.
[2] Gruenwald, Joerg, and Juergen Skrabal. 2003. “Kava Ban Highly Questionable: A Brief Summary of the Main Scientific Findings Presented in the ‘in Depth Investigation on EU Member States Market Restrictions on Kava Products.’” Seminars in Integrative Medicine 1 (4): 199–210. https://doi.org/10.1016/j.sigm.2004.01.001.

