verticity
I'm interested in things
I'm reposting this as it's own thread since I don't know if anyone will see it in it's original location.
I am questioning my sanity. On kava forums people have been referring to the kavalactones DHM and DHK as "double bonded" for awhile. But I have come to believe they are not, in fact, double bonded. Blasphemy!
I'm not a kava expert. I read about "double bonded" kavalactones here, and I believed it, too; it seemed to make sense that DHK and DHM are chemically distinct from the other KLs, evidenced by their heavy effects. But then I went back and looked at Lebot's book, and other academic sources, looked at the diagrams of the molecules, and I did not see these double bonds everyone was talking about, so I was like "what the.." and "but I thought..".
So first, let me explain what those pictures of molecules mean. They are diagrams of organic molecules, which means they are made up of carbon, hydrogen and oxygen atoms primarily, sometimes nitrogen or a few other things; But KLs are just carbon, hydrogen and oxygen. The letter "O" represents an oxygen atom. "C" represents carbon, and "H" represents hydrogen. But also, chemists are lazy, so they generally don't show all the carbons and hydrogens explicitly. Every vertex in a diagram like that represents a carbon atom. The hydrogens are usually not represented at all, but there are hydrogens attached to each carbon atom (that has less than 4 bonds to other atoms), so that each carbon atom has four bonds. It may be bonded to four other atoms, for example, here is methane (showing the hydrogens explicitly) because there is only one carbon atom):
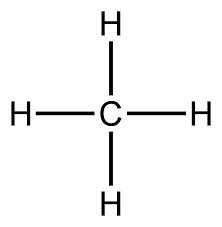
Methane is just a carbon bonded to 4 hydrogens. Like I said, the vertices of the diagrams can represent carbon atoms, so here is propane, explicitly showing the atoms:

(Obviously I suck as an artist) So propane is 3 carbons with attached hydrogens. Notice that each carbon atom is bonded to four other atoms. This is also propane:
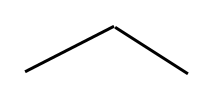
There is one carbon at the bend in the line, and one carbon each at the ends of the lines. The hydrogen atoms are implicit.
Now, carbon always wants to form 4 bonds, but some of those can be double bonds, Each double bond counts as two bonds (duh), so carbon can only bond to two other atoms if it is double bonded to something. Example, here is propene:
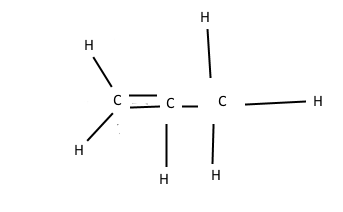
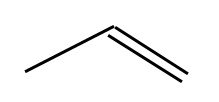
It has a double bond, represented by 2 lines. This is also propene:
Another thing to know is when you see a benzene ring which looks like this:
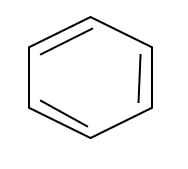
Those things that look like double bonds are actually not really double bonds, because the electrons are delocalized over the whole ring, so it's like the whole ring partakes of one big circular bond.
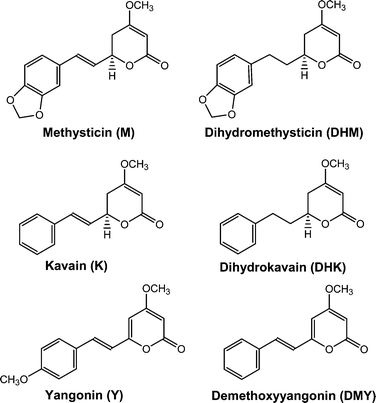
So finally we come to kavalactones. Here is dihydromethysticin:
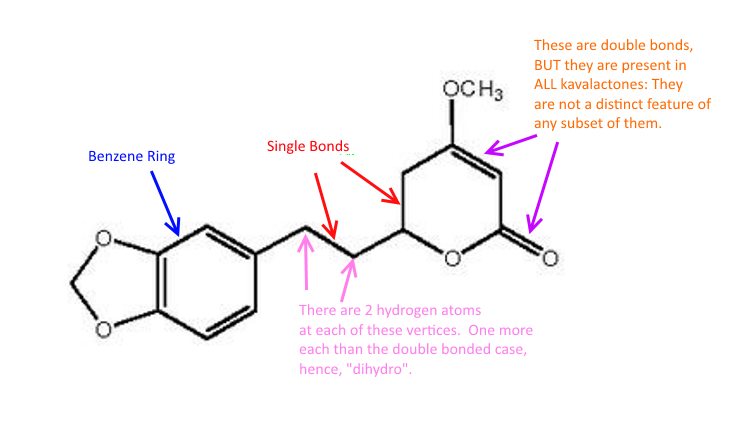
Lebot's book classifies KLs by whether they have single or double bonds at the locations with the red arrows above. Different KLs have different combinations of single/double bonds at those sites. It so happens that DHK and DHM have single bonds there.
Here is methysticin:
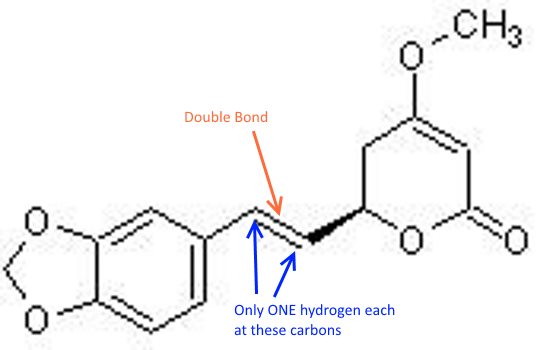
Crappy picture, but you can see Methysticin has a double bond where dihydromethysticin has a single bond, but they are otherwise identical.
If you look at the diagrams of Kavain and dihydrokavain, you'll see it's a similar story.
Did I just blow your mind?
I am questioning my sanity. On kava forums people have been referring to the kavalactones DHM and DHK as "double bonded" for awhile. But I have come to believe they are not, in fact, double bonded. Blasphemy!
I'm not a kava expert. I read about "double bonded" kavalactones here, and I believed it, too; it seemed to make sense that DHK and DHM are chemically distinct from the other KLs, evidenced by their heavy effects. But then I went back and looked at Lebot's book, and other academic sources, looked at the diagrams of the molecules, and I did not see these double bonds everyone was talking about, so I was like "what the.." and "but I thought..".
So first, let me explain what those pictures of molecules mean. They are diagrams of organic molecules, which means they are made up of carbon, hydrogen and oxygen atoms primarily, sometimes nitrogen or a few other things; But KLs are just carbon, hydrogen and oxygen. The letter "O" represents an oxygen atom. "C" represents carbon, and "H" represents hydrogen. But also, chemists are lazy, so they generally don't show all the carbons and hydrogens explicitly. Every vertex in a diagram like that represents a carbon atom. The hydrogens are usually not represented at all, but there are hydrogens attached to each carbon atom (that has less than 4 bonds to other atoms), so that each carbon atom has four bonds. It may be bonded to four other atoms, for example, here is methane (showing the hydrogens explicitly) because there is only one carbon atom):

Methane is just a carbon bonded to 4 hydrogens. Like I said, the vertices of the diagrams can represent carbon atoms, so here is propane, explicitly showing the atoms:

(Obviously I suck as an artist) So propane is 3 carbons with attached hydrogens. Notice that each carbon atom is bonded to four other atoms. This is also propane:

There is one carbon at the bend in the line, and one carbon each at the ends of the lines. The hydrogen atoms are implicit.
Now, carbon always wants to form 4 bonds, but some of those can be double bonds, Each double bond counts as two bonds (duh), so carbon can only bond to two other atoms if it is double bonded to something. Example, here is propene:

It has a double bond, represented by 2 lines. This is also propene:

Another thing to know is when you see a benzene ring which looks like this:

Those things that look like double bonds are actually not really double bonds, because the electrons are delocalized over the whole ring, so it's like the whole ring partakes of one big circular bond.
So finally we come to kavalactones. Here is dihydromethysticin:

Lebot's book classifies KLs by whether they have single or double bonds at the locations with the red arrows above. Different KLs have different combinations of single/double bonds at those sites. It so happens that DHK and DHM have single bonds there.
Here is methysticin:

Crappy picture, but you can see Methysticin has a double bond where dihydromethysticin has a single bond, but they are otherwise identical.
If you look at the diagrams of Kavain and dihydrokavain, you'll see it's a similar story.
Did I just blow your mind?


 , I want resolution!
, I want resolution!